drug dilution guidelines
For pediatric cardiac cases. If you need to compound a drug for injection accuracy aseptic techniques are to be followed.
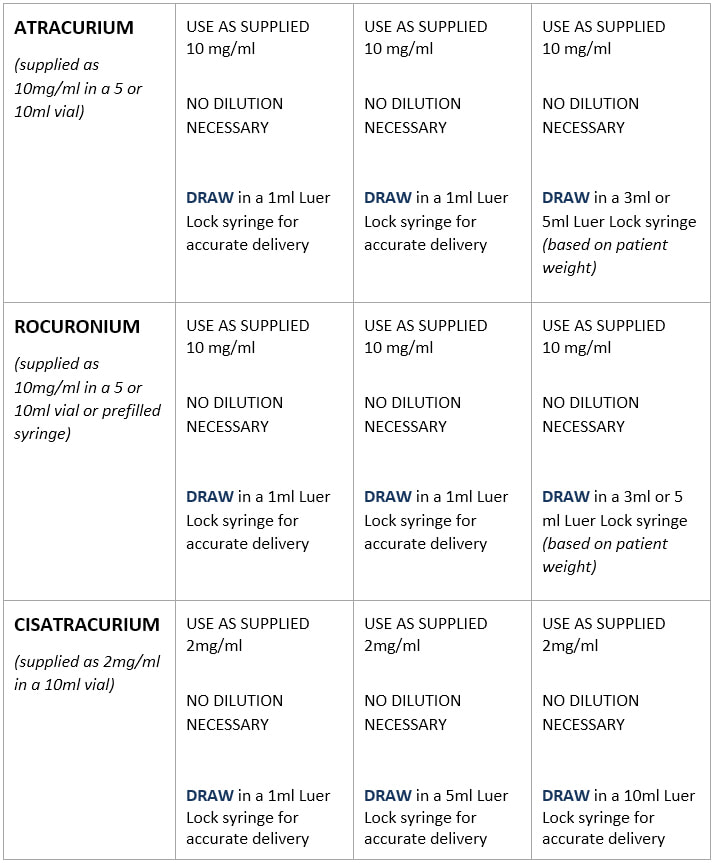
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
Some ampoules may require the application of a file to snap the neck although the dot marker has minimised this.

. Particularly the determination of steady-state binding affinities is widely used in biochemistry and pharmacology for drug discovery processes. All diluted drugs must be labeled with the following information. Enter the amount of substance used in mL.
UMMC Pediatrics Dilution Protocol for Antimicrobials Antifungals and Antivirals. Dilution of IV push medications should be done only when either required by the manufacturer based on peer-reviewed evidence or by institutional guidelines. Add 20mL from 1 vial dobutamine to 425mL normal saline.
Heparin GTN dobutamine dopamine. Each monograph contains stability data administration guidelines and methods of preparation. Incubate the plate at 37ºC 5 CO2 overnight before adding the drug dilutions.
Dose of 100 mcg and more. Eppendorf tubes or screw cap test tubes do not. The availability of this guideline will assist in the expansion of quality clinical care pharmacy services throughout MOH facilities.
6242019 6 The concentration of medications is the amount of drug diluted in a given volume of IV solution usually measured in units micrograms mcg milligrams mg or grams gm. - Tap the top of the ampoule to allow any trapped drug to drain into the bottom. Diluents should be sterile pharmaceutical preparations such as normal saline for injection bacteriostatic saline for injection or lactated Ringers solution for injection.
The length of time is either. The information provided in this guideline mainly extracted from the package insert for the brand of injectable drugs used and valid at the time. Name of person who made the dilution.
By convention 1 in 50 means 1g in 50mL. Dilution series are a common method in different scientific fields to generate standard curves for calibration of instruments or for the evaluation of the bioactive potential of diverse substances. Doses have varying units of measurement.
Select the use type by clicking the drop-down box and selecting Dilution. Prepare a solution at 50 mcgmL Take 1 mL of 250 mcg mL solution and mix with 4 mL NS to obtain a final concentration of 50 mcgmL or 250 mcg 5 mL. Dilute 50mg in 500ml ns 10ml 1mghr start infusion at 15mlhr 15mghr increase 15mlhr every 15min aim decrease diastolic bp 90-100 caution when.
Manufacturers recommend do not mix with any other drugs Reconstituted vial stable for 24 hours in the fridge or 8 hours at room temperature. Find the concentration of the diluted product as a percentage strength a ratio strength and an amount strength expressed as mgmL. This reference contains standard dilutions including IV admixture drug concentration infusion volumes and infusion rates.
No respondents described a dilution process that would result in a specific concentration eg add 4 mL of diluent to 1 mL of drug to equal xx mg5 mL. To enable screen reader support press CtrlAltZ To learn about keyboard shortcuts press Ctrlslash. Turn on screen reader support.
Prepare Drug Dilutions This step requires calculations of course you can calculate. May rarely cause allergic reactions. Expiration date which is thirty 30 days after the dilution date provided this does not pass either the expiration date of the drug listed on the original container or the sterile diluent see 5 below.
Hydralazine for preeclampsia eclampsia method 1. This is usually based on previous reports related to the compounds anticancer efficiencytoxicity range. A Abciximab reopro Acetaminophen Acetazolamide Diamox Acetylcysteine Acyclovir Zovirax ADAKVEO crizanlizumab.
This guideline was prepared to facilitate the healthcare staffs in preparing the dilutions of drugs which are available in the Ministry of Health MOH drug lists. No dilution required 250 mcgmL Dose of 999 mcg and less. 100mL of a 1 in 50 wv solution is diluted to 1000mL.
If you were logging a usage you would have selected Administration. Reperfusion arrhythmias increased risk of bleeding. Dilution should never be done with a manufactured prefilled saline flush syringe.
The dose of the medication is the amount of drug ordered for infusion over a specific length of time. Dilution reconstitution or the safe rate of administration of IV push medications Risks Associated with Drug Labeling Packaging and Nomenclature IV medications that are prepared in empty sterile syringes but left unlabeled IV push medications that are prepared diluted reconstituted in commercially available syringes of 09 sodium. Smaller volume 625mL.
The compounded drugs container must be sterile and opaque if compounding light sensitive drugs. Intermittent infusion 50mg over 30minutes and then 35mg over. If there is 1g in 50mL there is 2g in 100mL.
Draw up 425mL normal saline in a 60 cc syringe then add 250mg 20mL from 1 vial of dobutamine to the syringe Dopamine 3200 mcg mL. Institutions should provide the appropriate diluent along with the drug to ensure safety. - Snap the top using a dot marker as a guide if appropriate.
Antimicrobial Antifungal and Antiviral Drugs in Pediatrics University Malaya Medical Center - Google Docs. - Cover the top of the ampoule with gauze. Select the substance by clicking the drop-down box and selecting the stock container ID the dilution is being made from.
Benchmade - diluents should not be used even if sterilized as these may contain pyrogens that cannot be removed by filtration or heating. Dilution Guideline for Injectable Drugs serves as a general reference for the healthcare professionals in the Ministry of Health on how to prepare dilution of certain injectable drugs before administering to the patient. As suggested by the variability of dilution methods and volumes less than half of respondents 43 reported organizational policies or guidelines on dilution.
You need to decide the testing range of your compound. IV slow push over at least 5 minutes Compatible with D5W NS. Dilute 50mg in 50cc ns 1mg 1ml start 5mlhr increase 1mlhr every 15-20min max infusion rate 10mlhr method 2.
Drugs requiring dilution are to be mixed with an appropriate diluent in a separate sterile container to reach required working concentration. Use the correct diluent. For adult cases ____________.
Most of the remaining.

Dilution Chart Magnet Plant Therapy
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices

Drug Dilutions Used For Intradermal Skin Testing Download Table

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram

Antimicrobial Susceptibility Testing Of Mycobacterium Tuberculosis Complex Isolates The Eucast Broth Microdilution Reference Method For Mic Determination Clinical Microbiology And Infection
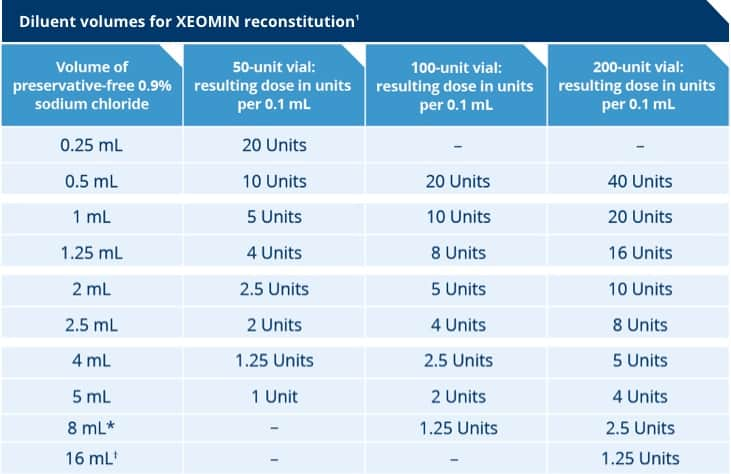
Reconstitution Dilution And Storage Xeomin
Wall Chart Drugs Given By Iv Push Or Rapid Administration In Adults Institute For Safe Medication Practices
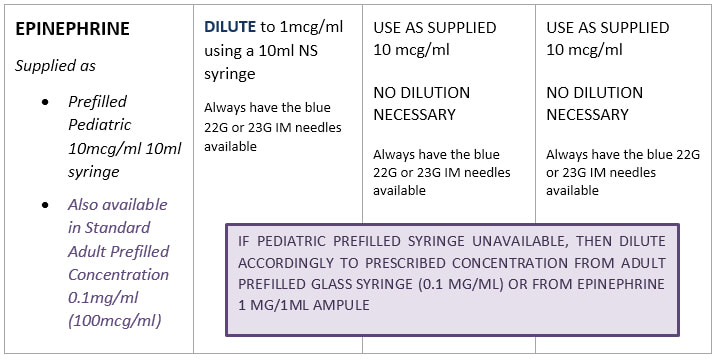
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices
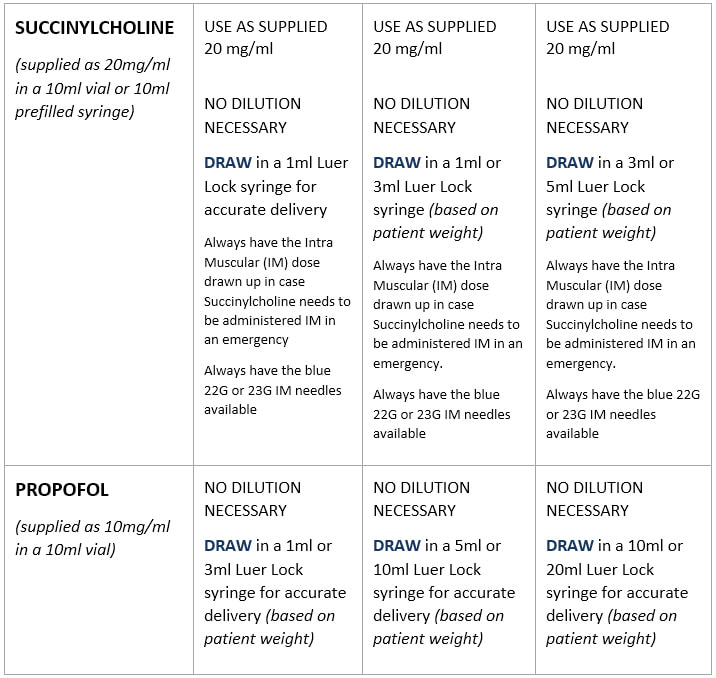
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
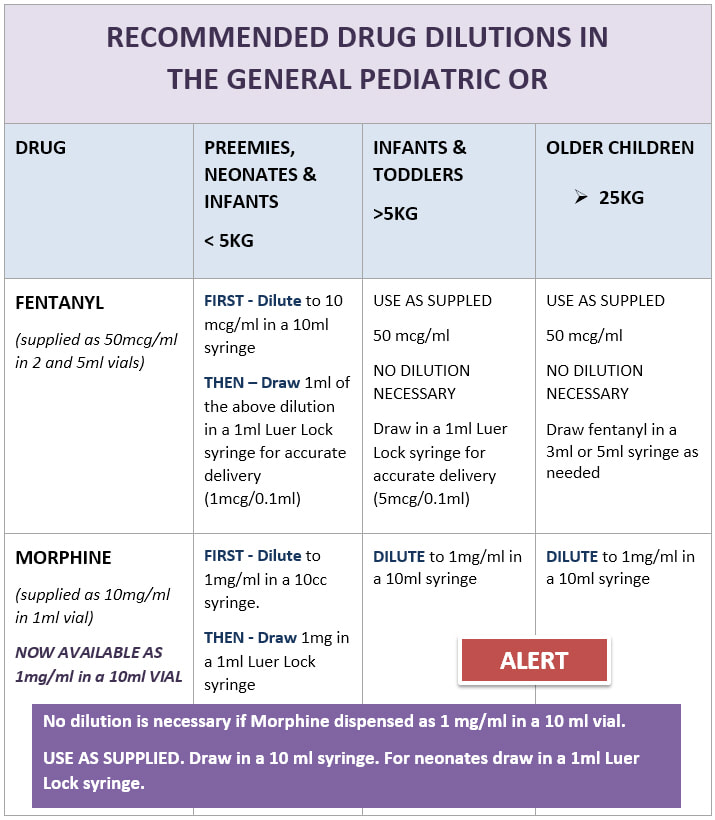
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Intravenous Iv Preparation And Infusion Guidelines Globalrph




0 Response to "drug dilution guidelines"
Post a Comment